See clinical trial results for patients who received continuous intravenous (IV) epoprostenol plus conventional therapy*
*Compared to those who received conventional therapy alone.
By clicking “CONTINUE” below, you will be taken to a website that may contain links or references to other websites to which our Privacy Policy may not apply. We encourage you to read the Privacy Policy of every website you visit.
You are solely responsible for your interactions with such websites.
The information contained in this section of the site is intended for US healthcare professionals only.
Click the appropriate link below.
Please see full Prescribing Information, including BOXED WARNING for OPSUMIT®.

Please see full Prescribing Information, including BOXED WARNING for TRACLEER®.

Please see full Prescribing Information for VENTAVIS®.

Please see full Prescribing Information for UPTRAVI®.
Clicking CONTINUE below will take you to the selected site, the content for which Janssen is not responsible and to which this Privacy Policy does not apply. We encourage you to read the Privacy Policy of every online service you visit.
CONTINUE*Compared to those who received conventional therapy alone.
These results are from a 12-week, prospective, open-label, randomized trial. All patients were either New York Heart Association (NYHA) Functional Class III or IV, except for 5 NYHA Functional Class II patients.
Epoprostenol plus conventional therapy
(n=56)†
Conventional therapy alone
(n=55)†
In a 12-week, prospective, open-label, randomized trial:
| • | The primary endpoint was a change from baseline in exercise capacity as measured by the 6-Minute Walk Test |
| • | Other major study objectives were changes in cardiopulmonary hemodynamics from baseline, including cardiac index, mean pulmonary arterial pressure, and pulmonary vascular resistance1 |
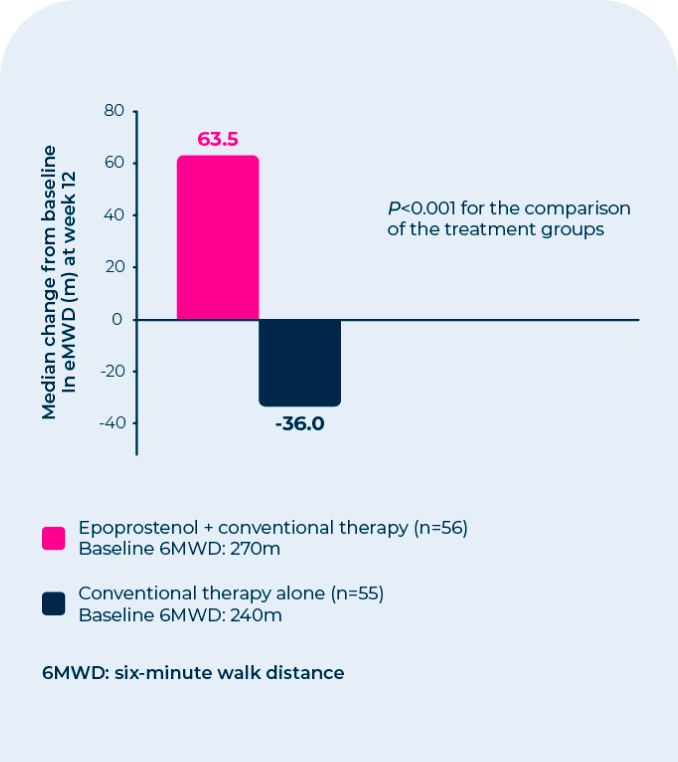
| • | Statistically significant improvement was observed in exercise capacity, as measured by the 6-Minute Walk Test |
| • | Improvements were apparent in some patients at the end of the first week of therapy |
No statistical difference in survival over 12 weeks was observed in PAH-SSD patients treated with epoprostenol as compared to those receiving conventional therapy alone.
| Baseline Values | Mean Change From Baseline at End of Treatment Period‡ | |||
|---|---|---|---|---|
| Parameter | Epoprostenol Plus Conventional Therapy (n=56) | Conventional Therapy Alone (n=55) | Epoprostenol Plus Conventional Therapy (n=50) | Conventional Therapy Alone (n=48) |
| CI (L/min/m2) | 1.9 | 2.2 | 0.5‡ | -0.1 |
| mPAP (mmHg) | 51 | 49 | -5‡ | 1 |
| mRAP (mmHg) | 13 | 11 | -1‡ | 1 |
| PVR (Wood units) | 14 | 11 | -5‡ | 1 |
| mSAP (mmHg) | 93 | 89 | -8‡ | -1 |
| Baseline Values | |
|---|---|
| CI (L/min/m2) | |
| Epoprostenol Plus Conventional Therapy (n=56) | 1.9 |
| Conventional Therapy Alone (n=55) | 2.2 |
| mPAP (mmHg) | |
| Epoprostenol Plus Conventional Therapy (n=56) | 51 |
| Conventional Therapy Alone (n=55) | 49 |
| mRAP (mmHg) | |
| Epoprostenol Plus Conventional Therapy (n=56) | 13 |
| Conventional Therapy Alone (n=55) | 11 |
| PVR (Wood units) | |
| Epoprostenol Plus Conventional Therapy (n=56) | 14 |
| Conventional Therapy Alone (n=55) | 11 |
| mSAP (mmHg) | |
| Epoprostenol Plus Conventional Therapy (n=56) | 93 |
| Conventional Therapy Alone (n=55) | 89 |
| Mean Change From Baseline at End of Treatment Period‡ | |
|---|---|
| CI (L/min/m2) | |
| Epoprostenol Plus Conventional Therapy (n=50) | 0.5‡ |
| Conventional Therapy Alone (n=48) | -0.1 |
| mPAP (mmHg) | |
| Epoprostenol Plus Conventional Therapy (n=50) | -5‡ |
| Conventional Therapy Alone (n=48) | 1 |
| mRAP (mmHg) | |
| Epoprostenol Plus Conventional Therapy (n=50) | -1‡ |
| Conventional Therapy Alone (n=48) | 1 |
| PVR (Wood units) | |
| Epoprostenol Plus Conventional Therapy (n=50) | -5‡ |
| Conventional Therapy Alone (n=48) | 1 |
| mSAP (mmHg) | |
| Epoprostenol Plus Conventional Therapy (n=50) | -8‡ |
| Conventional Therapy Alone (n=48) | -1 |
| • | Cardiac Index by 26% |
| • | Mean Right Atrial Pressure by 8% |
| • | Mean Pulmonary Arterial Pressure by 10% |
| • | Pulmonary Vascular Resistance by 36% |
| • | Mean Systemic Arterial Pressure by 9% |
In a 12-week, prospective, open-label, randomized trial, statistically significant differences in hemodynamic parameters were observed in patients who received epoprostenol plus conventional therapy.§
These hemodynamic parameters generally worsened in the group receiving conventional therapy alone.
§Compared with those who received conventional therapy alone.The most common and dose-limiting adverse events during dose initiation and escalation (≥1%) were:
| • | Flushing (58%) |
| • | Headache (49%) |
| • | Nausea/vomiting (32%) |
| • | Hypotension (16%) |
| • | Anxiety/nervousness/agitation (11%) |
| • | Chest pain (11%) |
| • | Dizziness (8%) |
| • | Bradycardia (5%) |
| • | Abdominal pain (5%) |
| • | Musculoskeletal pain (3%) |
| • | Dyspnea (2%) |
| • | Back pain (2%) |
| • | Sweating, dyspepsia, hypesthesia/paresthesia, tachycardia (1%), respectively |
Adverse events occurring in patients with PAH-SSD with ≥10% difference between epoprostenol and conventional therapy alone were:
| • | Flushing (23% vs 0%) |
| • | Hypotension (13% vs 0%) |
| • | Anorexia (66% vs 47%) |
| • | Nausea/vomiting (41% vs 16%) |
| • | Diarrhea (50% vs 5%) |
| • | Jaw pain (75% vs 0%) |
| • | Pain/neck pain/arthralgia (84% vs 65%) |
| • | Headache (46% vs 5%) |
| • | Skin ulcer (39% vs 24%) |
| • | Eczema/rash/urticaria (25% vs 4%) |
Thrombocytopenia has been reported during uncontrolled clinical trials in patients receiving epoprostenol.
Potential adverse events from postmarketing evaluations include anemia, hypersplenism, pancytopenia, splenomegaly, and hyperthyroidism.
Although the relationship to epoprostenol administration has not been established, pulmonary embolism has been reported in several patients taking epoprostenol and there have been reports of hepatic failure.
References: 1. Badesch DB, Tapson VF, McGoon MD, et al. Continuous intravenous epoprostenol for pulmonary hypertension due to the scleroderma spectrum of disease: a randomized, controlled trial. Ann Intern Med. 2000;132:425-434. 2. VELETRI® (epoprostenol) for Injection Full Prescribing Information. Actelion Pharmaceuticals US, Inc.